Battery Types
Rechargeable batteries offer a wide range of characteristics. This article explores the most common types.
Lithium-Ion Battery (LiB)
Lithium-ion battery is used in a variety of applications, from small devices such as smartphones to those mounted on electric vehicles and aircraft. Its key feature is a high energy density, enabling smaller and lighter batteries for a given capacity.
Lithium Iron Phosphate Battery (LFP)
Lithium Iron Phosphate (LFP) batteries are a type of lithium-ion battery that uses lithium iron phosphate (LiFePO₄) as the cathode material. While their energy density is lower than that of ternary (NMC) lithium-ion batteries, their greatest advantage lies in their exceptional safety, with an extremely low risk of thermal runaway.
Sodium-ion Battery (SIB)
Sodium-ion batteries (SIBs) are a new type of rechargeable battery that use sodium--an abundant and low-cost resource--instead of lithium. Their basic operating principle is similar to lithium-ion batteries: charging and discharging occur via the movement of sodium ions (Na⁺) between the cathode and anode. Since they do not require rare metals like lithium or cobalt, they offer promising advantages in terms of cost and resource availability.
Nickel-Metal-Hydride Battery (NiMH)
Rechargeable batteries in standard AA and AAA sizes, such as "eneloop" and "Evolta," are predominantly nickel-metal-hydride (NiMH) batteries, although some older nickel-cadmium (Ni-Cd) types may still be found. However, because the circuit to prevent overcharging differs depending on the product, a dedicated charger is required to prevent liquid leakage and shortened lifespan.
Lead-Acid Battery
Another common type is the lead-acid battery. Lead (Pb) is used for the negative electrode, lead dioxide (PbO2) is used for the positive electrode, and dilute sulfuric acid is used as the electrolyte. It is widely used as the 12 V starter battery in cars and motorcycles, making it a very common and familiar type of storage battery.
Sodium-Sulfur (NaS) Battery
The NAS (sodium-sulfur) battery uses natrium (or sodium, Na) for the negative electrode and sulfur (S) for the positive electrode. Ford Motor announced the principle in 1967, and NGK was the first company in the world to commercialize this type of storage battery. Fine ceramics are used for the electrolyte that separates the two electrodes. Unlike ordinary storage batteries, Na and S used in the electrodes are liquids, and the fine ceramic electrolyte is solid.
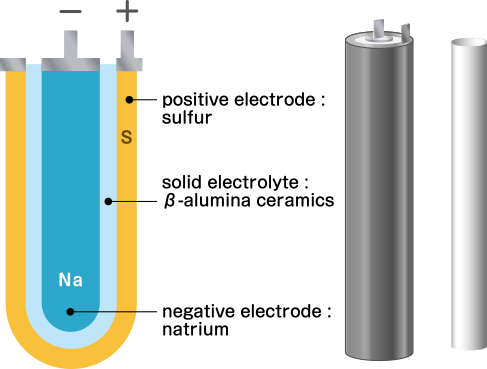
It is mainly attached to power plants that use natural energy such as solar power and wind power. It is used to adjust the amount of power supplied and to maintain the power quality.
However, NGK Insulators, Ltd., the world's sole manufacturer and distributor of NAS batteries, announced on October 31, 2025, in their "Notice Regarding Discontinuation of Manufacturing and Sales Activities of NAS Batteries" a decision to discontinue accepting new orders.
Redox Flow Battery
Finally, it is a redox flow battery. NASA announced the basic principle in the 1970s, and redox is a coined word combining reduction and oxidation. A redox flow battery cell consists of two electrodes separated by a central diaphragm, which allows only specific ions (such as hydrogen ions) to pass through. During operation, two different liquid electrolytes are circulated from external tanks to their respective electrodes by pumps. This process drives the redox reaction, enabling the battery to charge and discharge. Currently, systems in practical use primarily utilize vanadium-based solutions for both electrolytes. As the pentavalent vanadium ion becomes tetravalent in the positive electrode and the divalent vanadium ion becomes trivalent in the negative electrode, electrons can be released from the negative electrode and transferred to the positive electrode.
During charging, a reverse reaction occurs.
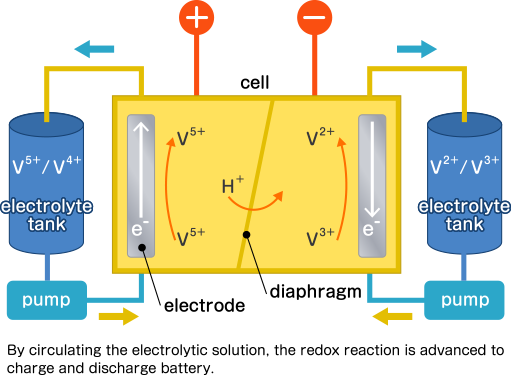
These are also installed in/used for power plants like NAS batteries.
Characteristics of Each Battery Type
Next, we introduce the features of each battery.
Lithium-Ion Battery (LiB)
Lithium-ion batteries have an electromotive force of about 3.7 V, and have the advantage of achieving high voltage independently. The electromotive force depends on the type of lithium oxide used for the positive electrode. Main materials used for the positive electrode are lithium cobaltate (LiCoO2), lithium manganate (LiMn2O4), lithium nickelate (LiNiO2), lithium iron phosphate (LiFePo4), and so on. Cobalt and manganese have a maximum voltage of 4.2 V. On the other hand, iron phosphates have a low voltage of around 3.6 V, but the charge/discharge cycle life is three to four times longer. Another advantage is that there is no memory effect.
However, there is a disadvantage because it uses Li with extremely high reactivity. Safety measures are important as with the NAS battery described later.
There are three types of lithium-ion batteries: cylindrical, square, and laminate types. Cylindrical types risk causing significant damage to the surroundings when it ruptures because internal gas pressure cannot easily escape. Compared to that, the square type expands easily, making it easier to release the internal pressure. While the pouch (laminate) type releases pressure most easily, this also means it is prone to swelling.
Should it become swollen, you should stop using it further.
Constant Current/Constant Voltage control is used for charge. This is the same principle as quick charging of lead-acid batteries described later. At first, charging with a fixed current (for example, current of 1C), but when the voltage reaches 4.2V, charging is changed with constant voltage. The current gradually decreases toward full charge.
However, lithium compounds are difficult to handle, and overcharging can cause serious accidents. Therefore, protection functions are required, such as stopping the charge when the cell voltage exceeds 4.30 ±0.05V.
Lithium Iron Phosphate Battery (LFP)
LFP batteries are increasingly recognized as a safe, long-lasting, and cost-effective energy storage solution. They are not only widely used in electric vehicles (EVs) and energy storage systems (ESS), but are also attracting attention as a replacement for traditional lead-acid batteries. Compared to lead-acid batteries, LFP offers higher energy density, lighter weight, and significantly lower self-discharge rates. These features make them ideal for applications such as uninterruptible power supplies (UPS), telecommunications equipment, forklifts, camper vans, and solar power storage.
With a cycle life of 2,000 to over 5,000 charge/discharge cycles and stable performance with minimal fire risk, LFP batteries provide outstanding reliability. They also do not require costly rare metals such as nickel or cobalt, contributing to lower material costs and supply chain stability. As a result, LFP batteries are poised to become a next-generation storage solution, forming the foundation of future mobility and energy infrastructure.
Although LFP batteries operate on the same basic principles as other lithium-ion batteries, their cell voltage and charging parameters differ. They follow the common CC-CV (constant current-constant voltage) charging method, but with a nominal voltage of 3.2V, a typical charge termination voltage of 3.6V, and a discharge cutoff voltage of approximately 2.5V -- generally lower than conventional Li-ion batteries.
Sodium-ion Battery (SIB)
Structurally and chemically, SIBs closely resemble lithium-ion batteries. However, by utilizing sodium--one of the most abundant elements on Earth--they present a more sustainable and potentially lower-cost alternative. Their nominal voltage ranges from approximately 2.3V to 3.0V, and although their energy density is lower than lithium-ion batteries, they are highly regarded for their safety and thermal stability. These characteristics make SIBs particularly well-suited for renewable energy storage and industrial applications that demand stable operation in harsh environments.
Development and commercialization of SIBs are accelerating, especially in China, with manufacturers such as CATL and Faradion already establishing mass production capabilities. While current adoption in EVs is still limited, future advancements in technology may position sodium-ion batteries as a viable substitute for certain lithium-ion applications.
Like lithium-ion batteries, sodium-ion batteries operate based on the movement of ions between electrodes, but their voltage characteristics differ. They also employ the CC-CV charging method, but typically with a nominal voltage of 2.3-3.0V, a charge termination voltage of 3.6-4.0V, and a discharge cutoff voltage of around 1.5-2.0V. Overall, the voltage range is slightly lower and broader than that of lithium-ion batteries.
Nickel-Metal-Hydride Battery (NiMH)
Nickel-metal hydride batteries have an electromotive force of 1.2 V, which is almost the same as nickel-cadmium batteries. Their environmental impact is low since they do not use cadmium. By using a hydrogen storage alloy for the negative electrode, hydrogen can be efficiently taken in and out, so the energy density is higher than NiCd despite the same voltage. They are widely available for consumer use.
On the other hand, the memory effect is a disadvantage. Therefore, it is necessary to be careful about charge/discharge, and if adding and repeating charge is repeated, the voltage drop will occur, and there is a high possibility that the voltage necessary to operate the device cannot be maintained.
Constant Current control is used for charge. The battery voltage rises toward the full charge, but the voltage drops when it is overcharged. Since it is necessary to stop charging this point, perform - ΔV (Negative Delta V) detection method on the control circuit. Absolute temperature control (dT/dt control) to detect temperature rise may be used together. This is also for NiCd.
Lead-Acid Battery
Lead-acid batteries have a nominal voltage of about 2.1 V per cell, which is relatively high. We cannot use applications where the number of charge/discharge cycles is large, but the advantage is that they can be easily obtained without memory effects. The commercially available 12 V battery is realized by connecting six storage batteries in series.
Being resistant to temperature changes is another advantage. In the case of automotive batteries, it is in the range of 80 degrees to -18 degrees to meet the discharge characteristic specifications.
In specific cases, -30 degrees are also available, but 25 degrees is recommended as the proper temperature. The reason is that when used in a low-temperature environment, the concentration of the electrolyte decreases as the discharge proceeds so that the electrolyte freezes and the discharge cannot be continued. Even if the electrolyte does not freeze, the battery's capacity is significantly reduced at low temperatures.
The disadvantage is that the allowable number of charge/discharge cycles is small, as described earlier. This causes the electrode plate to degrade by repeated expansion and contraction of the electrode by charge/discharge.
Constant Voltage control is used for charge. V taper control and Constant Current/Constant Voltage control are used when you want to charge quickly. In contrast to Constant Current/Constant Voltage control, which controls the current and voltage finely, the circuit design can be performed relatively inexpensively in the case of using V taper control because it only terminates charging if a sharp rise in voltage occurs.
NAS (sodium-sulfur) Battery
Inside the NAS battery, the following reaction occurs at the time of discharge.
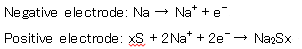
When sodium becomes a monovalent ion, it emits electrons, while sodium polysulfide is produced at the positive electrode. When charging, this is the reverse reaction.
NAS batteries have almost the same energy density as lithium-ion batteries and do not self-discharge. Another advantage is the ability to rapidly charge/discharge.
There is also a disadvantage. The largest one is the use of sodium with very high reactivity to the negative electrode, so there is a possibility of ignition. If there are holes or gaps even a little, they react with the water vapor in the air and generate heat. Therefore, when developing NAS batteries, it is necessary to conduct various safety performance confirmation tests such as external heating, submersion, dropping, and external short circuits.
Redox Flow Battery
The redox flow battery has a long charge/discharge cycle life of more than 10,000 times and has the advantage of being usable for over ten years. Electrolytes based on vanadium solutions are non-combustible, and battery operation is performed at room temperature. Therefore, there is no danger of ignition or explosion, and there is no need for heat resistance, so operation for more than 20 years is also possible.
Also, since the storage capacity is defined by the amount of electrolyte and the output is also defined by the size of the battery cell stack, it is possible to design these individually. This is also an advantage in design and development.
On the other hand, since miniaturization is impossible, it is limited to large facilities. This is because the weight energy density is only about 25 to 35 Wh/kg and only half to 1/8 of the 80 to 200 Wh/kg of lithium-ion batteries. Unless the energy density can be increased by devising the electrolytic solution, miniaturization is difficult.
| Battery type | Charge control mode |
|---|---|
| Lithium-ion Battery (LiB) Lithium Iron Phosphate Battery (LFP) Sodium-ion Battery (SIB) |
Constant Current/Constant Voltage controlCharging by constant current control up to a fixed voltage, and after reaching the set voltage value, charging with constant voltage control. Can be fully charged but requires fine control. 
|
Constant Current controlCharging by a fixed amount of current. In order to prevent overcharging, it is necessary to control when the voltage reaches a set voltage or to finish charging in a fixed time. 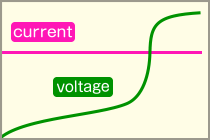
|
|
| NiMH Battery Ni-Cd Battery |
-ΔV (Negative Delta V) Detection MethodThis method utilizes the slight voltage drop that occurs when the battery becomes fully charged and enters an overcharge state. Charging is terminated when this voltage drop is detected. 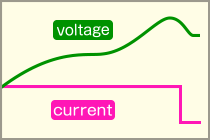
|
dT/dt Control (Temperature Rise Detection)This method utilizes the rapid temperature increase that occurs when the battery becomes fully charged. Charging is terminated if the rate of temperature increase (dT/dt) exceeds a set value. 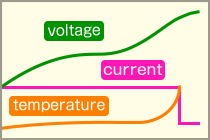
|
|
| Lead-acid Battery |
Constant Voltage controlCharge at a constant voltage. The current value gradually decreases toward full charge. 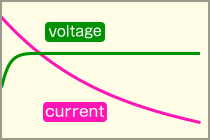 |
V taper controlCharging by constant current control to a fixed voltage and stop charging when it reaches the set voltage value. Easy to control, but may not achieve a full charge. 
|
|
Constant Current/Constant Voltage controlCharging by constant current control up to a fixed voltage, and after reaching the set voltage value, charging with constant voltage control. Allows for a full charge but requires precise control circuitry. 
|
Related Technical Articles
Recommended products
Matsusada Precision's products can be used in all kinds of rechargeable batteries and capacitors for development, evaluation, and test.
Reference (Japanese site)
- Japanese source page 「蓄電方法の種類と特徴」
(https://www.matsusada.co.jp/column/charge-sb.html) - 「充電用電池の基礎と電源回路設計」トランジスタ技術SPECIAL2013 Winter, No.121
(https://shop.cqpub.co.jp/detail/1402/) - NAS電池 - 日本ガイシ
(https://www.ngk.co.jp/product/nas.html) - レドックスフロー電池とは何ですか?
(https://batterybank.jp/question/system/redox.php) - バナジウムレドックスフロー電池の特長
(https://www.lesys.jp/redox/merit.php)(Dead link) - Vanadium redox battery
(https://everything2.com/?node=Vanadium+redox+battery) - 新しい電池の科学(講談社ブルーバックス)
(https://bookclub.kodansha.co.jp/product?item=0000194491)






