Battery Types and Problems
Countless electronic devices surround us in our daily lives. While large appliances like TVs and refrigerators draw power from wall outlets, portable devices--from smartphones and tablets to electric vehicles--rely on batteries. Batteries power a wide range of applications, from remote controls to industrial equipment. Recently, large-scale household storage batteries and V2H (Vehicle to Home) systems have gained attention for storing solar power for nighttime use.
Various types of batteries and rechargeable batteries are built-in these products and used properly according to the application. You can learn the types of batteries and the problems on this page.
Batteries are generally categorized into three types: primary cells (dry-cell batteries), secondary cells (rechargeable batteries), and fuel cells. Primary cells generate power through irreversible chemical reactions. Once the active materials are depleted, they cannot be reused and must be disposed of.
On the other hand, rechargeable batteries can be repeatedly charged and discharged. It also generates electrical power by chemical reactions, but it is different from primary cells in the reversibility of chemical reactions. After discharge, there is no substance that undergoes a chemical reaction, "charging" can be done to return from the state after reaction to the state before reaction.
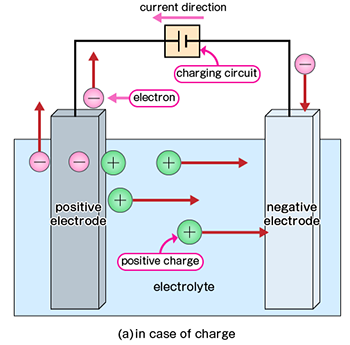
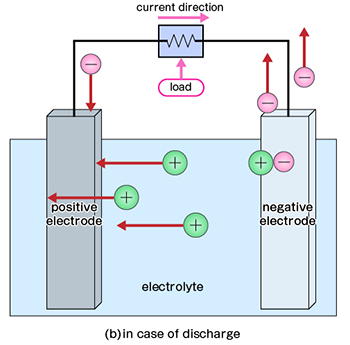
However, charge-discharge cycles have an upper limit and may cause a temporary voltage drop due to memory effects. Fuel cells generate electrical power by reacting hydrogen and oxygen. In this case, hydrogen is extracted from a hydrogen storage alloy or liberates hydrogen contained in hydrocarbons such as methane, ethane, propane, and butane in city gas and reacts with oxygen in the air. Therefore, if we can continue to provide hydrogen source materials, we can continue extracting electrical power.
Features of Each Battery
Let's introduce the features of each battery.
NiCd (Nickel-cadmium Battery), NiMH (Nickel-metal-hydride Battery)
Both NiCd and NiMH use nickel hydroxide for the positive electrode. NiCd uses cadmium for the negative electrode, NiMH uses a hydrogen storage alloy, and the electrolyte is an aqueous solution mainly composed of KOH (potassium hydroxide). Although both NiCd and NiMH batteries are suitable for high-power applications such as motors, NiCd usage has declined significantly. Due to environmental concerns regarding the toxic heavy metal cadmium, NiCd batteries have largely been superseded by Nickel-Metal Hydride (NiMH) technology. NiMH became the prevalent choice for common rechargeable batteries, and today, it is the technology used in most rechargeable AA and AAA batteries familiar to consumers, such as Panasonic's eneloop™ and EVOLTA™.
While NiMH batteries remain a reliable and popular choice for these applications, they face challenges such as the memory effect. In the broader market of portable electronics like smartphones and laptops, Lithium-ion (Li-ion) batteries have become the dominant technology due to their higher energy density and superior performance.
As a feature of NiMH, it is resistant to repeated charging and discharging and has high safety. On the other hand, NiMH has a strong memory effect, and there is a problem that the amount of electrical power that can be generated decreases as the battery charge is continued. To reset this memory effect, it must be fully discharged and then recharged.
LiB (Lithium Ion Battery)
Lithium-ion battery is used in various products such as laptop computers, smartphones, and tablets. Lithium-ion batteries (LiB) have become ubiquitous in modern electronics, powering everything from laptops and smartphones to tablets. It is also widely expected as a battery for electric vehicles. Since LiB has a very high energy density, about 2.5 times that of NiMH, it is an essential storage battery for reducing the weight of mobile devices such as mobile phones. Furthermore, they do not suffer from the memory effect, allowing for partial charging without degrading battery capacity. They also exhibit a very low self-discharge rate, retaining their charge for extended periods of non-use.
Table. Self-discharge Rate of Each Battery
| Battery type | Self-discharge rate [%/month] |
|---|---|
| Lead-acid battery | 3 to 20 |
| Nickel-cadmium battery | 20 to 45 |
| Nickel-metal-hydride battery | 15 to 40 |
| Lithium-ion battery | 1 to 5 |
However, because the energy density is high, even a slight voltage fluctuation results in overcharging, which leads to extremely shortening the battery life. In addition, if overcharging or over discharging is repeated, the internal pressure of the battery will rise, causing a burst or fire accident. You may have seen the news that a smartphone has exploded and caused a major burn, or regarding incidents involving battery fires on aircraft.
Solid-state Battery
One of the problems with the above batteries is "leakage". If it is left for a long time, the electrolyte contained in the battery may leak out. Leakage can cause a short circuit, and in some cases, can damage the internal circuitry. Leakage is a risk in traditional batteries that use liquid electrolytes. To address this issue, solid-state batteries utilizing solid electrolytes have been developed. The advantage is that it is easy to handle, and it is less likely to cause an accident.
Early prototypes of solid-state batteries demonstrated volumetric energy densities around 200 Wh/L. However, intensive research and development are currently underway, with goals to significantly increase this density to 400-600 Wh/L or even higher, which is crucial for extending the driving range of future electric vehicles.
<--On the other hand, there is also a drawback. The solid-state battery developed by Toyota for electric vehicles is a lithium-ion storage battery that uses a sulfide-based solid electrolyte and a layered oxide positive electrode, and currently has a volumetric energy density of only about 200 Wh/L. This is less than half. In order to extend the cruising distance, it is necessary to raise the volumetric energy density to 400 to 600 Wh/L, and its development is currently in progress.
-->Metal-air Battery
A metal-air battery uses oxygen in the air for the positive electrode and metal for the negative electrode. It is used for small and light electric appliances such as hearing aids and kitchen timers. Alkali metals such as Li, Na, and Ca and alkaline earth metals are chemically reacted with oxygen in the air to extract electricity.
Therefore, when not in use, keep it shielded to block oxygen and peel off the shield when it is used. Battery performance varies depending on the metal (element) used, and the merit is that it can be downsized because it does not require an electrolyte. On the other hand, it is difficult to develop as a rechargeable battery because metal causes dendrite growth at the time of charging, and an infrastructure for recycling is necessary.
Fuel Battery
Fuel batteries are fundamentally different from the rechargeable batteries introduced so far. Basically, you can think of it as the same as a generator. In other words, by continuing to provide some kind of fuel, the generator is continued generating electrical power.
While they may seem less familiar in daily life compared to standard batteries, fuel cells are becoming more common. Panasonic's "ENE-FARM" is a typical example of a residential fuel cell system. The hydrogen that is provided as fuel and the oxygen in the air react with each other to produce water and generate electrical power. Electrical power is generated by the reverse reaction of the water electrolysis experiment conducted in science experiments.
However, since hydrogen has a high reaction rate and needs attention in handling, for example, for a fuel battery considered for mounting on an electric vehicle, it should not be accumulated even if hydrogen leaks or hydrogen does not leak to the surroundings.
Double-layer Capacitor
It is a kind of capacitor that has become the focus of attention in recent years. Like a battery, it is composed of an electrode and an electrolyte, but when a voltage is applied between the electrodes (to the extent that the electrolyte does not decompose), there is a region where the charge of the electrode and the opposite sign increases around the electrode. Then, it can store potential like a capacitor, and this is called an electric double-layer capacitor.
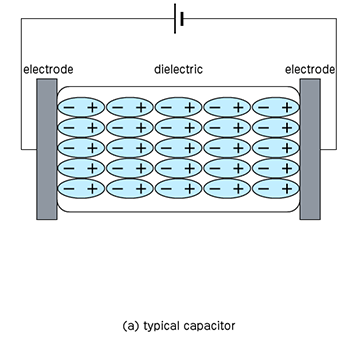
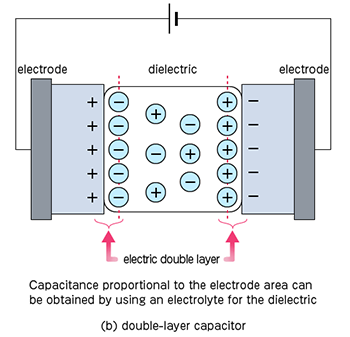
Similar to rechargeable batteries, it can be repeatedly charged and discharged, but it is characterized by its high charge and discharge speed because it only forms the electric double-layer. However, the energy density is smaller compared to rechargeable batteries. On the other hand, the energy density is higher than ceramic capacitors, but the rated voltage is lower.
Related Technical Articles
Recommended products
Matsusada Precision's battery cycle testers, DC power supplies, and bidirectional power supplies (regenerative power supplies) are used in evaluation tests and production lines for electric batteries such as lithium-ion batteries and capacitors.
Reference (Japanese site)
- Japanese source page 「電池の基本 種類と特徴を知る」
(https://www.matsusada.co.jp/column/kind-of-cell.html) - 「Liイオン/鉛/NiMH蓄電池の充電&電源技術」トランジスタ技術SPECIAL 2016 Summer, No.135
(https://shop.cqpub.co.jp/detail/1942/) - リチウムイオン電池の基本
(https://pubdata.nikkan.co.jp/uploads/book/pdf_file4fa08a3c3ccbf.pdf) - トヨタの全固体電池 2025~30年EVが化ける
(https://www.nikkei.com/article/DGXMZO34637170X20C18A8000000/) - 燃料電池のしくみ
(https://panasonic.biz/appliance/FC/enefarm/about_fuelcells.html) - 電気二重層キャパシタの基礎(前編)
https://www.murata.com/ja-jp/products/emiconfun/capacitor/2015/03/24/20150324-p1(Dead link)






