Mechanism of Discharge
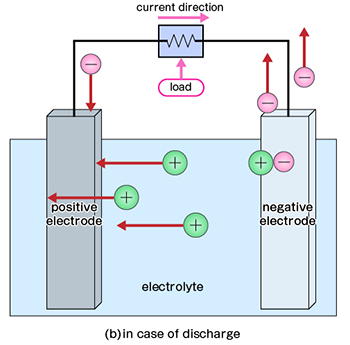
Discharging is the process of drawing electricity from a battery. In both primary and rechargeable batteries, electrochemical reactions generate a flow of electrons. This section explains how electricity is produced through these internal chemical reactions.
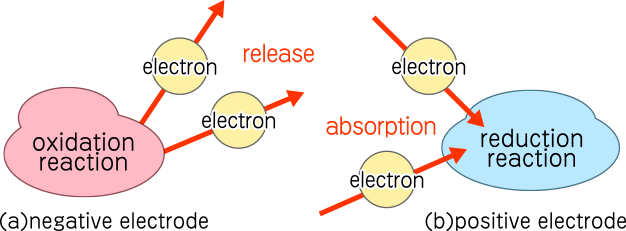
A battery consists of positive and negative electrodes. At the negative electrode, an oxidation reaction occurs, releasing electrons. Conversely, at the positive electrode, a reduction reaction occurs, absorbing electrons. The surplus electrons generated at the negative electrode flow through an external circuit to the positive electrode to balance the electron deficiency caused by the reduction reaction.
The specific redox reactions depend on the electrode materials and the electrolyte. These reactions continue until the active materials required for the reaction are depleted. In other words, the battery continues to generate electricity until it is fully discharged.
Mechanism of Charge
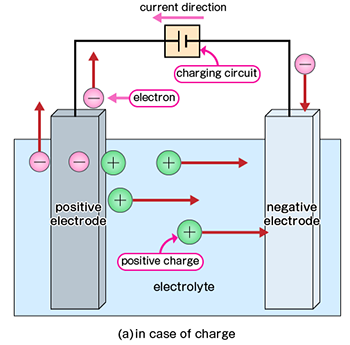
Charging is the process of restoring energy to a rechargeable battery for reuse. In a fully discharged state, the substances within the battery are in chemical equilibrium. However, by applying an external voltage, it is possible to reverse the chemical reactions, extracting electrons from the positive electrode and returning them to the negative electrode to restore the battery's original state.
During charging, the reactions are reversed compared to discharging: an oxidation reaction occurs at the positive electrode, and a reduction reaction occurs at the negative electrode. Electrons supplied by an external power source drive this reverse electrochemical reaction. Primary batteries, however, cannot be charged because their chemical reactions are irreversible, or because recharging is economically or structurally impractical. Therefore, they are designed for single-use applications.
Chemical Reaction and Electrical Characteristics during Charge and Discharge
Now, we introduce examples of chemical reactions during charge/discharge and electrical characteristics of various batteries in terms of "electrochemistry".
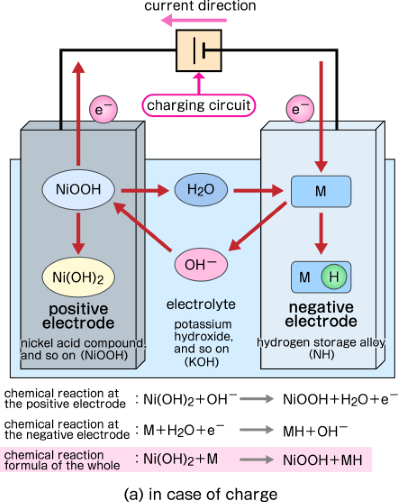
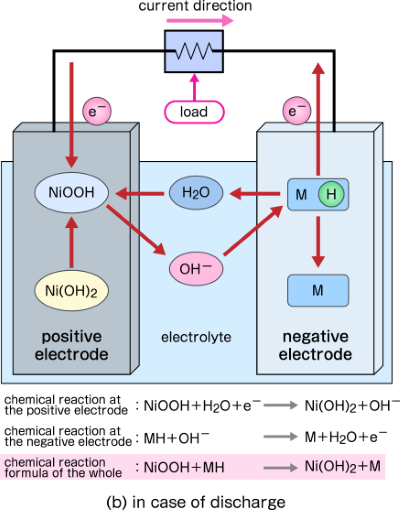
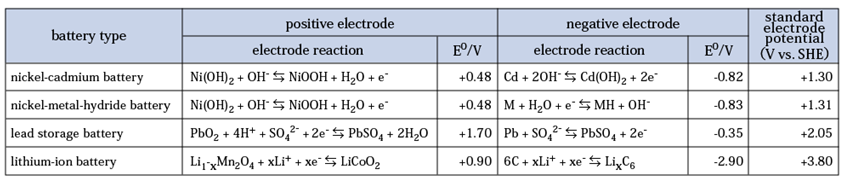
First, we explain the chemical reaction inside the storage battery, taking NiMH (nickel-metal hydride battery) as an example. A nickel acid compound is used for the positive electrode, and a hydrogen storage alloy is used for the negative electrode in NiMH. During charging, water molecules are generated from hydroxide ions at the positive electrode. Water molecules are decomposed into hydrogen atoms and hydroxide ions at the negative electrode, and hydrogen atoms are stored in a hydrogen storage alloy. The chemical reaction formula is as follows (M means hydrogen storage alloy).
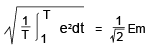
During discharging, hydroxide ions are generated from water molecules at the positive electrode, and they move from the positive electrode to the negative electrode in the electrolyte. Hydroxide ions transferred to the negative electrode receive hydrogen ions from the hydrogen storage alloy and return to water molecules. The chemical reaction formula is as follows.
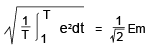
If this reaction is written in the electrochemical equilibrium formula, it becomes as follows.
The electrical characteristics of a battery are largely determined by the standard electrode potential E0 of its materials. The amount of energy generated depends on the electrochemical properties of the specific battery chemistry.
The theoretical electromotive force (EMF) is defined by the difference in electrical potential between the positive and negative electrode materials. This is derived from the standard electrode potential relative to the Standard Hydrogen Electrode (SHE).
The theoretical electromotive force is defined by the difference of electrical potential generated by the combination of the positive and negative electrode materials. This is the standard electrode potential. Then the energy of electrons generated at each pole is defined by the potential measured from SHE (Standard Hydrogen Electrode). "vs. SHE" means "SHE standard".
For example, in the case of a lithium-ion storage battery, if you use lithium cobaltite (LiCoO2) for the positive electrode and carbon for the negative electrode to extract electrons from Li, the difference of electrical potential with SHE is +0.87 V for the positive electrode and -2.83 for the negative electrode. The standard electrode potential is 0.87 - (-2.83) = 3.7 V vs. SHE.
Similarly, 1.32 V vs. SHE for NiCd (nickel-cadmium) batteries and 1.55 V vs. SHE for NiMH batteries. However, the EMF of the NiCd battery and NiMH battery is about 1.2 V, which is a little lower than the theoretical values.
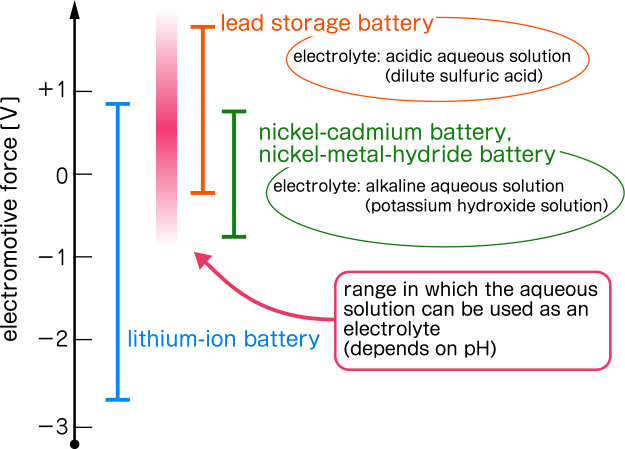
In the case of lead-acid batteries that are often used in automotive batteries, lead dioxide (PbO2) is used for the positive electrode and lead (Pb) for the negative electrode. Then the standard electrode potential of the positive electrode SHE standard is 1.70, and the negative electrode is -0.35, it will be about 2.0 V vs. SHE. This value almost agrees with the nominal value of the electromotive force of the lead-acid battery.
The standard electrode potentials of each battery are summarized in Table 1.
How can the electromotive force be increased? Since the potential of Lithium (-3.0 V vs. SHE) is close to the theoretical limit, increasing the single-cell voltage implies raising the potential of the positive electrode. Alternatively, higher voltages can be achieved by connecting multiple battery units, known as "cells," in series.
For example, a standard 12 V automotive lead-acid battery consists of six 2 V cells connected in series. Similarly, laptop computers often use battery packs consisting of multiple lithium-ion cells connected in series; for instance, three cells are connected to achieve a 10.8 V drive voltage.
Memory Effect
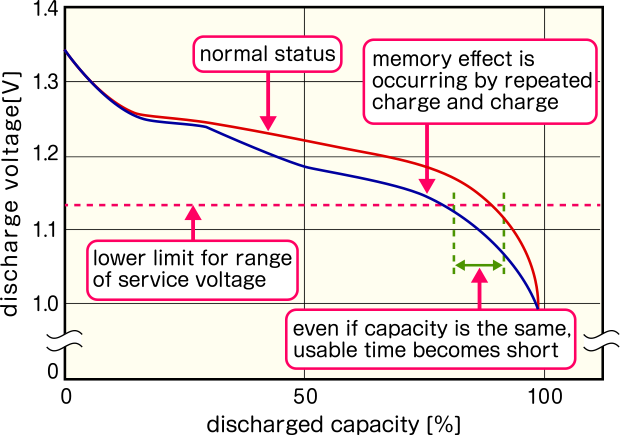
The "memory effect" is a phenomenon observed in NiCd and NiMH batteries where the discharge voltage drops if the battery is recharged before being fully discharged. The battery appears to "remember" the previous discharge level, reducing the available capacity for subsequent use. This can cause issues in high-voltage devices, such as digital cameras, which may not operate correctly due to the voltage drop. While the effect can often be resolved by fully discharging and recharging the battery, the precise electrochemical mechanism remains a subject of study.
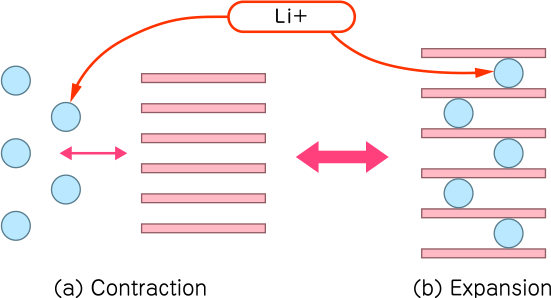
On the other hand, lithium-ion batteries have no memory effect and are suitable for repeated use. However, for both positive and negative electrodes, an intercalation reaction occurs in which Li+ enters and leaves the gap of the electrode structure material. This causes the electrode material to expand and contract slightly due to charge and discharge. But it is more stable than other batteries.
Lithium-ion Batteries and Safety
Lithium-ion batteries generally do not exhibit the memory effect and are suitable for repeated partial charging. In these batteries, an intercalation reaction occurs where lithium ions ($Li^+$) move in and out of the electrode structure. This causes the electrode material to expand and contract slightly during charge and discharge cycles.
While the intercalation process is generally stable, repeated overcharging or overdischarging can degrade the materials or cause metallic lithium deposition (dendrites). This degradation can lead to gas generation or internal short circuits, causing the battery pack to swell and, in extreme cases, resulting in thermal runaway or combustion.
Related Technical Articles
Recommended products
Matsusada Precision's battery cycle testers, DC power supplies, and bidirectional power supplies (regenerative power supplies) are used in evaluation tests and production lines for electric batteries such as lithium-ion batteries and capacitors.
Reference (Japanese site)
- Japanese source page 「充電・放電時に二次電池内部では何が起こっているか?」
(https://www.matsusada.co.jp/column/secondary-battery.html) - 「充電用電池の基礎と電源回路設計」トランジスタ技術SPECIAL2013 Winter, No.121
(https://shop.cqpub.co.jp/detail/1402/) - 電気化学便覧 第6版
(https://www.maruzen-publishing.co.jp/item/b294140.html) - 電子移動の化学 -電気化学入門
(https://www.asakura.co.jp/detail.php?book_code=14593)






