Toward an Economy That Stores Electricity
Recently, "Society 5.0" has become a keyword of the policy in Japan. The society of hunting-gathering was set as Society 1.0. After that, it is said that Society 5.0 will come through the agrarian society, the industrial society, and the information society. Society 5.0 is premised on communication technology such as 5G, collection and storage of information by IoT and cloud, and use of information by AI
Considering the progress of societies, there was a change: "Store resources and use them when you need them." First, food could be stored. Flood control stabilized the food that could only be harvested by the whimsical nature, such as the Fertile Crescent and the Nile basin in Egypt. For that purpose, in addition to flood control, storage and calendar played an important role.
Speaking of storage, grains can be stored for several years, and if processed into bread or the like, they can be immediately supplied with energy to move people. It can provide a long-term supply of the active energy that people cultivate until they get a harvest the following year. Grain was considered to be a very important element of the storage method in terms of storing human activity energy.
To make grains that can be stored, we selected varieties, stored them, and repeated breeding. Finally, it is said that civilization has begun by finding grains that can produce many times of energy work compared to labor for farming.
This time, we will talk about storage, so we decided to proceed with the theme of hydrogen as one of the energy storage methods. Hydrogen is one of the means to store electric energy made from renewable energy for a long time and to generate electric energy immediately.
Electrolysis and Fuel Cells
When it comes to storing electricity, the first thing that comes to mind is batteries or rechargeable batteries. In particular, rechargeable batteries that can be repeatedly charged and discharged are used in various fields, from smartphones and PCs to electric vehicles and aircraft.
Batteries are fine if they are stored for a short period. However, if you try to store it for a long time, it will deteriorate the performance over time, causing a voltage drop and more. When it comes to a portable storage method that can be stored across seasons and that can be moved around, fuel cells are the best choice, instead of primary or rechargeable batteries. Fuel cells, in particular, can generate electricity permanently by continuing to feed in positive and negative electrode materials.
The fuel cell was invented by Sir Humphry Davy (1778-1829), a British chemist and inventor.

Davy is also known for discovering new elements by electrolyzing various aqueous solutions using Volta batteries. Specifically, he found out reactive sodium and potassium.
Since he was mainly studying "Electrodynamics", which is science when electricity flows through a substance, he made various batteries by combining various metal plates in the process. Also, a paper called "On Some Chemical Agencies of Electricity" published in 1806 spread the knowledge about the subsequent chemical reactions.
After that, British scientist William Robert Grove (1811-1896) made the fuel cell. In 1839, he made a prototype of a fuel cell called a "gaseous voltaic cell". The fuel cell had platinum for the electrodes and dilute sulfuric acid for the electrolyte. It extracted electric power from hydrogen and oxygen, and then, the electric power electrolyzed water.
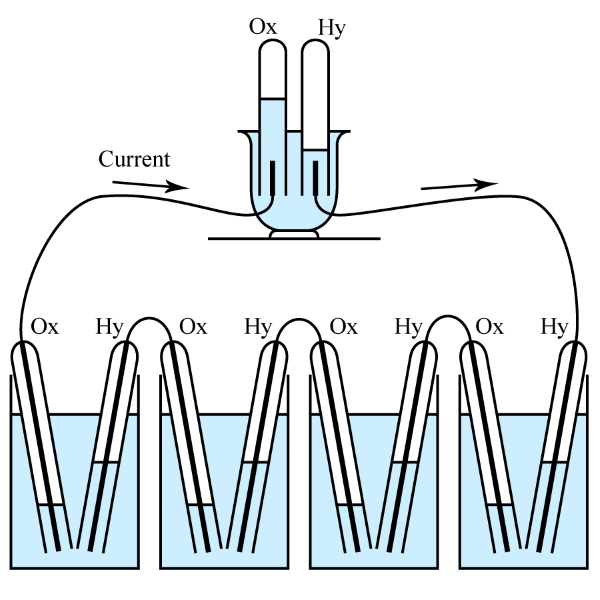
In fact, even in today's fuel cells, the basic chemical reaction for extracting electricity is the production of water by hydrogen and oxygen. In other words, electricity is taken out by performing the reverse reaction of electrolysis of water using a catalyst. Therefore, oxygen in the air is generally used as the positive electrode material (oxidizer), and the substance after the reaction is discharged as water vapor (negative electrode material: carbon dioxide depending on the fuel combination). Power generation stops when the negative electrode material is exhausted, so it can be said that it is similar to a primary battery.
Francis Thomas Bacon (1904-1992), also a British engineer, brought this fuel cell to a practical level. It is said to be a descendant of the famous philosopher Francis Bacon. In 1959, Bacon and his team developed the alkaline electrolyte fuel cell. Finally, this battery has greatly contributed to the Apollo 11 NASA's lunar landing. Incidentally, the world's first commercialized fuel cell was manufactured by General Electric Company (GE) and adopted in NASA's Gemini project.
Since then, fuel cells have continued to be improved in efficiency and ease of use. Currently, the research and development project of the following four methods are underway.
- Polymer Electrolyte Fuel Cell (PEFC)
- Phosphoric acid fuel cell (PAFC)
- Molten carbonate fuel cell (MCFC)
- Solid Oxide Fuel Cell (SOFC)
For example, in PEFCs, the positive electrode uses oxygen in the air, the negative electrode uses hydrogen, and an ion exchange membrane is used as the electrolyte. The following reactions occur with platinum as a catalyst at each electrode.
- positive electrode: O2 + 4H+ + 4e- → 2H2O
- negative electrode: H2 → 2H+ + 2e-
We are trying to create a hydrogen economy by using hydrogen as fuel. It is expected to be used in mobile terminals, household power supplies, and electric vehicles.
Energy Can Be Stored with Hydrogen (Because of a Hydrogen Economy)
As mentioned above, various media have been developed as fuel cell media. However, there is no doubt that hydrogen is a typical medium these days.
As a method of extracting hydrogen, a method of using hydrogen itself, a method of extracting hydrogen from a hydrocarbon such as methane, and a method of extracting hydrogen from a compound containing a large amount of hydrogen such as alcohol are considered. Alcohol is easy to handle, but currently mainstream is hydrogen and hydrocarbon.
Hydrocarbons are gases such as city gas. Therefore, the advantage is that there are already distribution channels. However, since carbon remains, this will be emitted as carbon monoxide and carbon dioxide.
Toyota, which is an automobile manufacturer, was particular about using hydrogen because it does not emit carbon dioxide. Only water is discharged, so it is clean for the environment. In addition, the amount of energy per volume is about eight times that of lithium-ion batteries, and the amount of energy per weight is 100 times or more, so it is extremely efficient.
In fact, in Germany, a "Power to Gas business" is underway to store and distribute energy by electrolyzing water using electricity generated from natural energy such as wind power to extract hydrogen and store it.
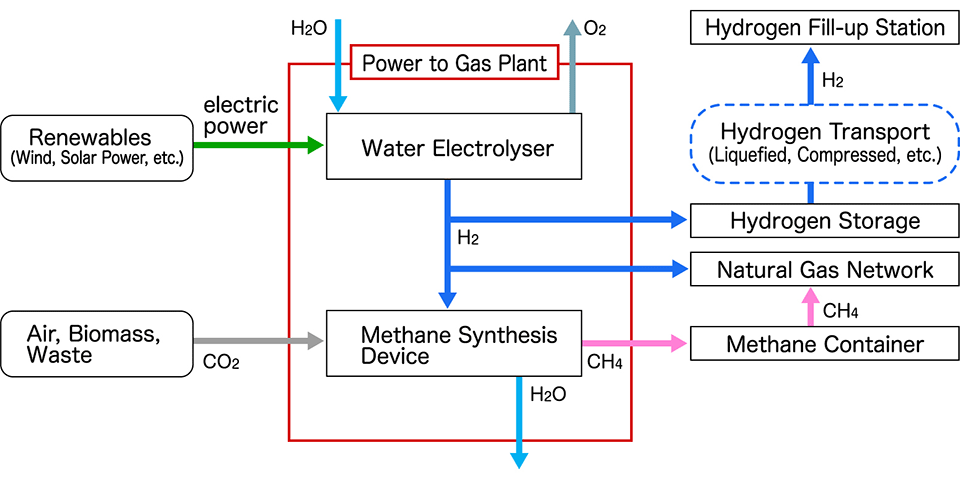
Currently, the size of the water electrolysis (Electrolyser) is being increased, but the cost of manufacturing, transportation, and storage is not clear, so it is necessary to consider at what scale it will be profitable in the future. In fact, a method of transporting it through a methane synthesis device and using a natural gas pipeline is also being considered.
In Japan, the Agency for Natural Resources and Energy has released a document "About hydrogen production, transportation and storage". According to this, in addition to the method of electrolyzing water to obtain hydrogen using electric power from natural energy, the officials are paying attention to the following two methods.
- Hydrogen generated when reforming fossil fuels such as oil and natural gas
- Hydrogen generated as a by-product from steelworks, chemical plants, etc.
They are also focusing on a method of using a photocatalyst.
However, hydrogen has its problems. Since hydrogen has a high rate of oxidation reaction, it is easy to extract electric power. On the other hand, if the control is wrong, it will be oxidized at once. Many people must have learned it in science class, with bringing hydrogen to fire and making a "quick pop" sound. Doing the same thing with a large amount of hydrogen could result in an explosion.
Another problem is that hydrogen is a gas and it is difficult to reduce its volume. Although there is also a method of making liquid hydrogen by compression and cooling, the huge amount of energy is needed to make liquid hydrogen, and also the cost of the storage equipment is very expensive.
Therefore, engineers are trying to use a method of storing hydrogen in a special alloy called "hydrogen-storing alloy". This alloy has a void that allows hydrogen to enter in the crystal structure and can exist to some extent with stability. The feature is that it can occlude and store hydrogen hundreds to over a thousand times its own volume in a stable manner.
The energy per weight is lower than that of hydrogen as it is, but offers a higher volumetric density than compressed hydrogen gas, making it suitable for specific applications. Therefore, long-distance transportation will likely utilize gas-related infrastructure such as pipelines, and hydrogen will be distributed at the end by using a hydrogen storage alloy.
Related Technical Articles
Recommended products
Matsusada Precision's products for electric power solution
Reference (Japanese site)
- Japanese source page 「水素社会への道を作ったデービーとベーコン」
(https://www.matsusada.co.jp/column/h_society.html) - 電気(コトバンク)
(https://kotobank.jp/word/電気-102136) - Tom Bacon(Little Shelford History)
https://sites.google.com/site/littleshelfordhistory/little-shelford-people/tom-bacon - 高効率水素吸蔵合金
(https://www.inpit.go.jp/blob/katsuyo/pdf/chart/fkagaku29.pdf) - 自立型再エネ水素発電設備導入ガイドライン(宮城県 環境生活部 再生可能エネルギー室)
https://www.pref.miyagi.jp/uploaded/attachment/731353.pdf - 「エネルギーの人類史」
バーツラフ・シュミル著,塩原通緒訳,青土社刊 - 水素の製造、輸送・貯蔵について(資源エネルギー庁)
https://www.meti.go.jp/committee/kenkyukai/energy/suiso_nenryodenchi/suiso_nenryodenchi_wg/pdf/005_02_00.pdf






