What is X-Ray Fluorescence?
When a material is irradiated with high-energy X-rays, inner-shell electrons within its atoms are ejected. To regain stability, an electron from a higher-energy outer shell fills the vacancy. This transition releases an X-ray with an energy level equal to the energy difference between the two shells. This emission is known as "X-ray fluorescence" (XRF).
The energy difference between the inner and outer shells is unique to each element, resulting in X-ray fluorescence with element-specific energy signatures. Generally, electron binding energy is highest in the shell closest to the nucleus (K shell), followed by the L and M shells. Consequently, fluorescence emitted during a transition to the K shell (e.g., from the L shell) is called Kα, while a transition from the M shell is called Kβ. These transitions to the inner shells produce higher energy X-rays compared to transitions involving only outer shells.
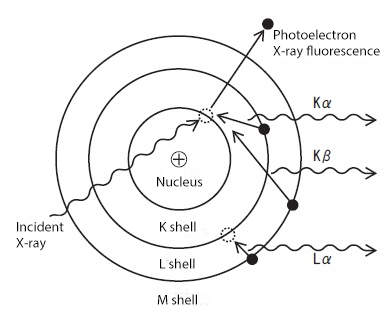
Principle of Measurement
Since the energy of fluorescent X-rays is unique to each element, analyzing the fluorescence spectrum of a sample allows for the identification of its constituent elements (qualitative analysis). Furthermore, the intensity of the spectrum is proportional to the element's concentration, enabling the determination of how much of each element is present (quantitative analysis).
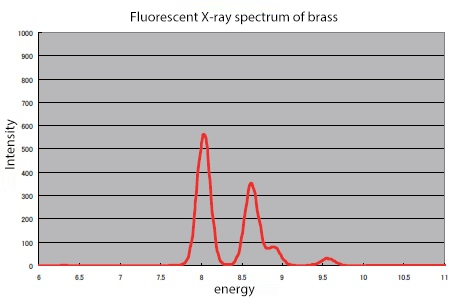
Figure 2 Fluorescent X-ray spectrum (Sample)
Analysis Equipment
X-ray fluorescence analyzers are broadly categorized into two types:
Wavelength Dispersive X-ray Fluorescence (WDX): These systems use Soller slits to collimate fluorescence emissions and spectroscopic crystals to separate wavelengths. WDX analyzers offer high detection sensitivity and superior energy resolution, making them ideal for applications requiring high precision.
Energy Dispersive X-ray Fluorescence (EDX): Typically equipped with a semiconductor detector, EDX systems can directly discriminate X-ray energy levels. This allows for compact designs, rapid analysis, and simultaneous multi-element detection.
For both WDX and EDX systems, the stability and low-noise performance of the high-voltage power supply are critical for ensuring measurement accuracy and repeatability.
Quantitative Analysis
Quantitative analysis determines the concentration of elements within a sample. In X-ray fluorescence analysis, two primary methods are used:
Calibration Curve Method: A standard method that determines the concentration of an unknown sample by comparing it to standard samples with known concentrations.
Fundamental Parameter (FP) Method: A theoretical approach that calculates composition based on the measured X-ray intensity and the physics of X-ray generation. This method is suitable for samples where standard samples are unavailable or the composition is unknown.
Sample Preparation Method
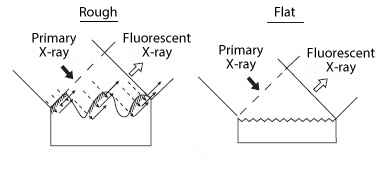
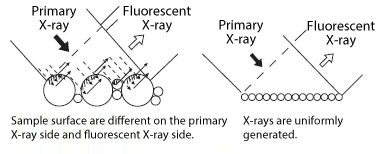
Although X-ray fluorescence analysis allows a variety of samples, such as solids, liquids, and powders, to be analyzed non-destructively, there is a possibility that the analysis results may differ depending on the shape of the sample or how the sample is placed in the container. (Figure 3) Therefore, the following points are important when preparing samples.
Points for preparing samples
- Prepare homogeneous samples
- Prepare the standard sample and each sample to be measured in the same way
- Avoid contamination
(a) Bulk sample
(b) Powder sample
Figure 3 Effects of specimen surface and granularity (particle size)
Regulations for Various Hazardous Substances
Strict management of hazardous chemical substances is a growing environmental priority. Regulations such as the RoHS directive have been established to prevent the spread of harmful chemicals. Consequently, there is a high demand for X-ray fluorescence analyzers (XRF) capable of performing quick and easy screening analyses.
* Revisions may be made to the legal interpretations of these regulations. The statements here do not represent the positions of official institutions. Be sure to refer to the original text of applicable laws and regulations.
RoHS Directive
The RoHS (Restriction of Hazardous Substances) directive was issued by the EU in 2003 and came into force in July 2006. To ship products to EU member countries, the content of restricted hazardous substances must remain below specified limits (Table 1).
XRF analyzers are non-destructive and offer rapid measurement capabilities, making them an ideal choice for screening these substances. Note that hexavalent chromium is detected as total chromium, and PBB/PBDE are analyzed as total bromine.
| Substance | Limit |
|---|---|
| Lead (Pb) | 1000ppm or less |
| Mercury (Hg) | 1000ppm or less |
| Cadmium (Cd) | 100ppm less |
| Hexavalent chromium (Cr6+) | 1000ppm or less |
| Polybrominated biphenyl (PBB) | 1000ppm or less |
| Polybrominated diphenyl ether (PBDE) | 1000ppm or less |
| Bis(2-ethylhexyl) phthalate (DEHP) | 1000ppm or less |
| Butyl benzyl phthalate (BBP) | 1000ppm or less |
| Dibutyl phthalate (DBP) | 1000ppm or less |
| Diisobutyl phthalate (DIBP) | 1000ppm or less |
Soil Contamination Countermeasures Act
(Note: This section refers to Japanese law. If aimed at a global audience, context is helpful.) In Japan, the Soil Contamination Countermeasures Act was enacted to prevent health hazards caused by soil contamination. Among the hazardous substances specified in this law, Class 2 heavy metals can be effectively analyzed using X-ray fluorescence.
The specified substances and their reference values are shown in Table 2. An official quantification method has been established only for total arsenic and total lead (JIS K 0470).
| Substance | Limit Standard |
|---|---|
| Lead (Pb) | 150 mg/kg |
| Cadmium (Cd) | 150 mg/kg |
| Hexavalent chromium (Cr6+) | 250 mg/kg |
| Arsenic (As) | 150 mg/kg |
| Mercury (T-Hg) | 15 mg/kg |
| Selenium (Se) | 150 mg/kg |
Regulations Regarding Toys
Toy safety is regulated by Part 3 of the EU Toy Safety Directive (EN71 Part 3), which limits the migration of heavy metals in toys designed for children. In Japan, Toy Safety Standards (ST-2002) were established, with regulation values for lead added in 2006.
These ST standards include the Food Sanitation Act, EN71, and other regulations as inspection items.
| Element | Regulation Value | |
|---|---|---|
| Excluding clay and finger paint |
Clay and finger paint | |
| Lead (Pb) | 90 ppm | 90 ppm |
| Mercury (Hg) | 60 ppm | 25 ppm |
| Cadmium (Cd) | 75 ppm | 50 ppm |
| Hexavalent chromium (Cr6+) | 60 ppm | 25 ppm |
| Barium (Ba) | 1000 ppm | 250 ppm |
| Arsenic (As) | 25 ppm | 25 ppm |
| Selenium (Se) | 500 ppm | 500 ppm |
| Antimony (Sb) | 60 ppm | 60 ppm |
Halogen-Free Measures
Halogen compounds have been used widely in electronic materials and other products as fire retardant materials. However, because they generate harmful gas when burned (dioxin in particular can be produced at high temperatures) and for environmental concerns such as preventing the destruction of the ozone layer, there is now a strong need to switch to halogen-free materials. However, there is no clear definition of "halogen-free".
For example, the Japan Electronics Packaging and Circuits Association (JPCA) has established regulation values for halogen content in printed wiring as a JPCA standard (Test Method for Halogen-Free Materials JPCA-ES01). It defines the total amount of chlorine (Cl) and bromine (Br) contained in epoxy resin and phenolic resin used in printed wiring boards. (Table 4)
This standard has also been adopted for IEC standard 61249-2-21 (International Electrotechnical Commission) and IPC standard 4101B (Association Connecting Electronics Industries).
| Material | Limit standard |
|---|---|
| Chlorine (Cl) | Chlorine (Cl): ≤ 900 ppm, Bromine (Br): ≤ 900 ppm, Total (Cl + Br): ≤ 1500 ppm |
| Bromine (Br) |
Related Technical Articles
Recommended products
Matsusada Precision delivers mission-critical high-voltage power solutions for analytical X-ray applications, including XRF and XRD. Our X-ray power supplies are engineered for the exceptional stability, low ripple, and compact design required for high-precision analysis.





