What is plasma?
Plasma is a state of matter in which energy is higher than gas. In general, there are three states of matter: solid, liquid, and gas. As the temperature rises, the solid becomes liquid and becomes gas.
For example, water becomes a solid called ice in the crystalline state, becomes liquid water when the temperature is raised, and becomes a gas called water vapor when the temperature is further raised. Thus, the energy possessed by a substance determines its state of matter.
Matter usually has electrons moving around its nucleus. An atomic nucleus has a positive charge, consisting of protons and neutrons. In contrast, since electrons have a negative charge, they are attracted to the nucleus by the Coulomb force and are always in motion around the nucleus.
However, when the temperature of the gas is further raised to a state of extremely high energy of about several thousand degrees Celsius, the electrons orbiting around the nucleus are separated (ionized) from the atom and become unstable. This state is plasma.
In an ionized and unstable state, the plasma emits light and electromagnetic waves and appears to shine because it releases energy and tries to return to a stable state. In addition, it is extremely easy for electric current to flow, and it is also characterized by the fact that the movement of electrons increases due to the electromagnetic force.
Although plasma seems to be a special state, it is often observed in nature. Thunder and aurora are two examples of this phenomenon. Industrially, it is also used in the manufacture of fluorescent lamps, plasma torches, and semiconductors.
Plasma in semiconductors
The following are three examples of the use of plasma in semiconductor manufacturing.
(1) Film Deposition (Plasma Enhanced Chemical Vapor Deposition, PECVD)
One of the most critical processes in semiconductor manufacturing is "film deposition," where a thin film is formed on a wafer. A specialized, high-quality deposition technique is called "epitaxial growth," where a new single-crystal layer is grown on top of a single-crystal substrate, inheriting its crystal structure. This process is essential for manufacturing high-performance semiconductor devices.
Chemical Vapor Deposition (CVD) is a representative method used for such film deposition, including epitaxial growth. Among CVD techniques, Plasma Enhanced Chemical Vapor Deposition (PECVD) utilizes plasma to facilitate the reaction.
In a PECVD system, the source gas supplied over the substrate is turned into plasma using direct current (DC), radio frequency (RF), or microwaves. This activates neutral excited particles, causing a chemical reaction on the substrate to deposit the generated material and form a thin film. This technique is widely used for depositing films like silicon nitride (SiN) and silicon oxide (SiO₂).
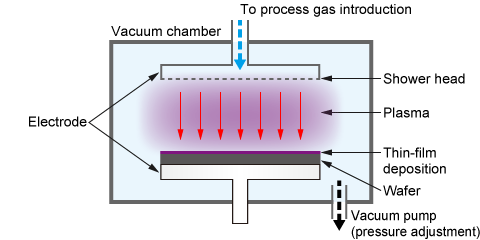
The main advantage of PECVD over conventional thermal CVD methods (often used in an epitaxial growth system) is its ability to form films at lower temperatures. This low-temperature process allows for deposition on substrates with complex topographies or those that cannot withstand high heat.
(2) Plasma dry etching
Etching is the process of carving grooves and patterns on the surface of a substrate. Conventionally, the wet etching method using an etching solution is used. However, in recent years, dry etching using etching gas or ions has become mainstream.
Plasma dry etching is a technology that performs etching by scraping the surface of the substrate with plasma. It is also called chemical-physical etching technology. As in plasma CVD, a gas is flowed onto the surface of the substrate, and the gas is turned into plasma. At this time, ions collide with the substrate to promote a chemical reaction with the substances contained in the plasma. This makes it possible to precisely scrape the surface of the substrate on an atomic scale. Dry etching generally includes Reactive Ion Etching (RIE) using vacuum discharge plasma such as Inductively Coupled Plasma (ICP) and Capacitively Coupled Plasma (CCP).
Unlike wet etching, no waste liquid is generated, so in addition to being a clean processing method, more accurate processing than wet etching is possible.
Deeper etching can also be used to separate semiconductor chips, known as plasma dicing. For dicing, please refer to "Back-End Semiconductor Manufacturing Process".
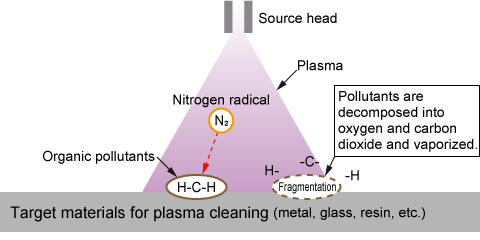
(3) Plasma cleaning
Plasma cleaning is a cleaning technology that decomposes and vaporizes organic substances such as oil adhering to the surface of the substrate with plasma. In addition to being a dry and clean method that does not use water or cleaning solutions, it also provides a high level of cleaning that leaves no residue.
Plasma cleaning can also make the surface of the treated object hydrophilic by breaking molecular bonds and decorating it with hydroxyl groups. In the semiconductor manufacturing process, it is also used to add hydrophilicity to the patterned PDMS (dimethylpolysiloxane) surface in order to improve pattern adhesion.
Is the flame plasma?
Burning candles and firewood raises a flame. In fact, it is often said that this flame is plasma. However, the brightness of the flame can be explained by something other than plasma origin.
When you burn a fuel such as a candle, the carbon contained in the fuel combines with oxygen in the air. The energy generated during oxidation is emitted as blue light from where the oxidation is occurring.
On the other hand, the brightest and shining part of the flame is due to the combustion of soot, which is the fuel that cannot be completely oxidized and is rolled up by the reaction. This is because any substance causes a phenomenon called "blackbody radiation" that emits yellowish light when heated to 1,000 °C or higher.
So what is it that makes it a plasma? In a flame, it is possible to discharge over a longer distance than usual. This meets the characteristic environment of plasma, where electricity flows easily.
In addition, the phenomenon that shadows are formed when light is applied to a flame also applies to the characteristic that light is absorbed or scattered when light collides with electrons moving in plasma. It is thought that the ionized fuel around the flame is in a plasma state and wraps around it.
However, some theories argue that a flame is not a "plasma" because its energy level is considered too low to meet the formal definition of plasma. In other words, although the flame is a type of plasma, there is no certainty that it meets the definition. It's a little interesting that the flame, which has been a familiar existence that has supported human life for a long time, is still being discussed as "What exactly is it?"
Reference (Japanese site)
- SAMCO社 - 「半導体製造装置入門 - プラズマとは」
(https://www.samco.co.jp/company/primer/2011/03/post_3.php) - SAMCO社 - 「半導体製造装置入門 - プラズマを技術に活かす」
(https://www.samco.co.jp/company/primer/2011/03/post.php) - SAMCO社 - 「半導体製造装置入門 - 身近なプラズマ」
(https://www.samco.co.jp/company/primer/2011/03/post_2.php) - P.R.A社 - 「プラズマの原理 - 半導体におけるプラズマとは」
(https://www.pra.jp/techinfo/plasma)
Related Technical Articles
Recommended products
Matsusada Precision's products meet the stringent specifications required by semiconductor manufacturing and are used in plasma processes.






