
Difference between radioactivity, radiation, and radioactive materials
Many of us have heard the words radioactivity, radiation, and X-rays before. Perhaps you may have a somewhat scary image of them. However, radioactivity and radiation are familiar to us and support our daily lives.
First, let's take a look at the meanings of the words radioactivity, radiation, and radioactive materials, and the differences between them.
- Ionizing radiation
- A general term for high-energy particle beams and electromagnetic waves capable of ionizing atoms. It is broadly categorized into two types: electromagnetic waves, such as gamma rays emitted from radioactive materials and X-rays generated by electron beam collisions; and particle radiation, such as alpha and beta rays.
- Radioactivity
- The ability of radioactive materials to emit radiation.
- Radioactive material
- A substance that emits radiation (sometimes described as radioisotope)
If we compare it to the relationship between a light bulb and light, radiation is the light emitted by the bulb, radioactive materials are the bulb, and radioactivity is the power to emit light. As a light bulb gets old, its power to emit light decreases. Therefore, the light becomes weaker or does not shine. The same is true for radioactive materials. As they continue to emit radiation, the radioactivity decays and the amount of radiation decreases.
Also, just as a light bulb is composed of parts that emit light and auxiliary parts that do not, in some cases radioactive materials are composed of elements that are radioactive and elements that are not radioactive.
Radiation is produced by the decay of atomic nuclei or changes in electron orbitals. It can be broadly divided into two types: particle radiation and electromagnetic radiation. Particle radiation involves the emission of particles such as protons, neutrons, and electrons (beta rays) from the atomic nucleus. Electromagnetic radiation, such as X-rays and gamma rays, is emitted as waves of energy.
Ionizing radiation is a type of electromagnetic wave that is characterized by its higher energy and shorter wavelength than radio waves, microwaves, and ultraviolet light, which are also members of the electromagnetic wave family. As a result, it has the power to ionize irradiated objects. Different types of radiation have different penetrating abilities, long and short wavelengths.
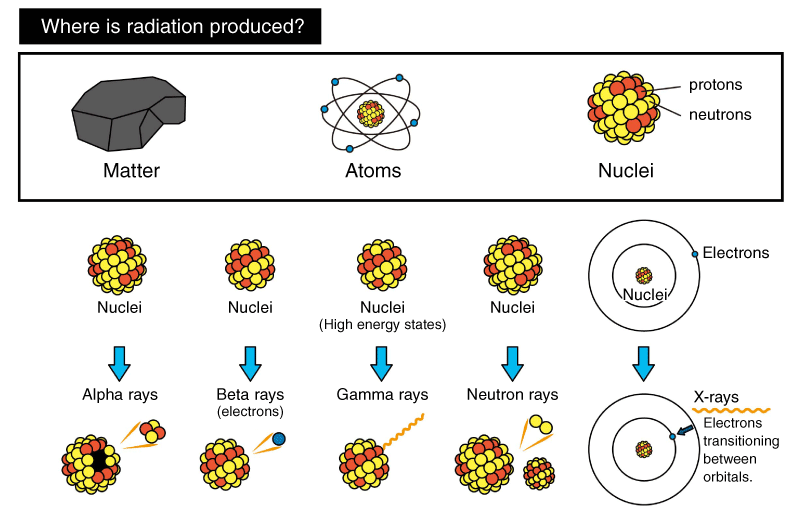
*Based on the Japanese Ministry of the Environment's website.
| Typical sources | Wavelength | Ionization density | Penetrating power | Characteristics | ||
|---|---|---|---|---|---|---|
| Electromagnetic radiation | Gamma rays | Cesium 137 | ~10pm | Low | High | Gamma rays are produced when the nucleus of an atom in a high-energy state releases energy and attempts to transition to a stable state. They are weakened by materials with high specific gravity, such as lead and concrete. |
| X-rays | X-ray tube | 1pm ~10nm | Low | High | X-rays are produced when electrons spinning outside the nucleus change their orbits, and the excess energy is emitted as electromagnetic waves. The more dense a material is, the more difficult it is to penetrate, a characteristic used in x-ray machines and x-ray non-destructive testing equipment. X-rays are weakened by materials with high specific gravity, such as lead and concrete. |
|
| Particle radiation | Alpha radiation | Radium 226 | High | Low | Alpha radiation is the result of the decay of an atomic nucleus, resulting in the ejection of a mass of two protons and two neutrons (helium nucleus). It can be shielded by a sheet of paper or skin. |
|
| Beta radiation | Iodine-131 | Low | Low | Beta particles (or beta rays) are high-energy electrons ejected from the nucleus of an atom during a process called beta decay. It is also called an electron beam. Because they are electrons themselves, they are easily absorbed by metals, and can be shielded by a thin piece of metal as small as a few millimeters. |
||
| Neutron radiation | Generated by accelerators | High | Low | Neutron radiation is made by taking neutrons, which make up the nucleus of an atom, and emitting them using an accelerator. They can be weakened by water or concrete, which contains a lot of hydrogen. |
||
Units of Radiation and Emission
There are three main units related to radiation: becquerel, sievert, and gray. Here is an explanation of what each of them means.
- Becquerel (Bq)
- A unit quantifying radioactivity. It represents the number of atomic nuclei that decay (disintegrate) per second.
- Gray (Gy)
- A unit that indicates the amount of energy that radiation gives to 1 kg of material.
- Sievert (Sv)
- A unit that indicates the amount of radiation applied to the human body (exposure dose).
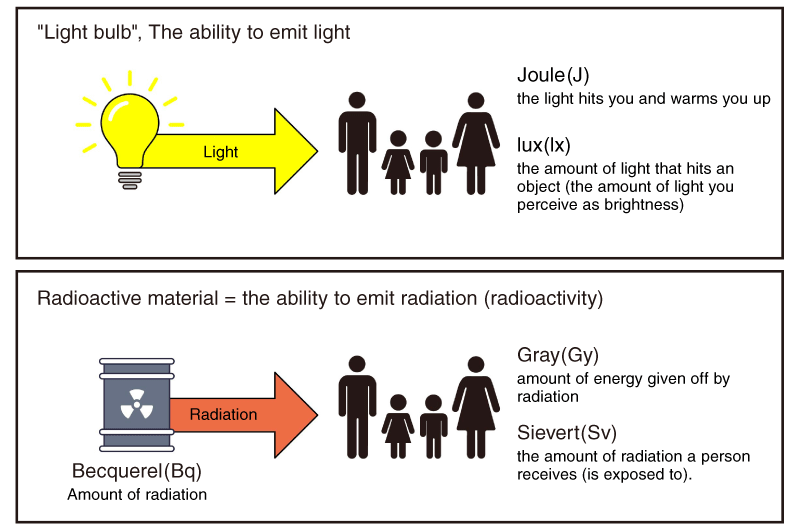
*Created based on the Japanese Ministry of the Environment website.
In the previous section, we compared radioactive materials to light bulbs and radioactivity to the ability to emit light. If we apply this example to units, the watt (W), which represents the intensity of light from a light source, becomes the becquerel (Bq), which represents the amount of radioactivity. The amount of light that hits an object and can be perceived as brightness, lux (Lx), becomes the amount of radiation a person is exposed to, sievert (Sv).
The feeling of warmth when exposed to sunlight is a little different from light, but it can be expressed in joules (J), which represents energy. The equivalent of this is the amount of energy that radiation gives to an object in grays (Gy).
The radiation that we receive (or are exposed to) does not only come from outside the body like light. If we inhale or ingest radioactive materials, we receive radiation from inside our bodies. For this reason, the amount of radiation a person receives, the sievert (Sv), is calculated by multiplying the amount of energy (Gy) absorbed by the human body by various coefficients.
Radioactive Half-Life
Radiation from radioactive materials (except X-rays) is produced when a radioactive element undergoes radioactive decay. As time goes by, the radioactive element changes into another element and becomes less radioactive. The period of time it takes for the radioactivity contained in a radioactive material to gradually decay and be reduced to half of its original level is called the half-life.
Radioactive decay occurs naturally, and the ease with which it occurs is determined by each radioactive element. The half-life does not change due to external physical or chemical effects and is determined for each element.
Radiocarbon dating takes advantage of the fact that each radioactive element has a different half-life. 14C, an isotope of carbon that is radioactive, is produced by cosmic rays that fall into the atmosphere, and carbon dioxide, which contains 14C, is retained in a certain amount in the body of plants and animals while they are alive but decreases according to its half-life after death.
Therefore, by comparing the amount of 14C in living plants and animals with the number of dead plants and animals, we can determine how long ago the plants and animals died, i.e., when they were alive.
Use of Radiation (X-rays)
Radiation is used in many places to support our lives. In particular, X-rays are used to irradiate objects and see through them, sSince bones and lesions are denser than surrounding tissues, they absorb more X-rays, appearing white on the detector or image.
In addition, X-ray systems are safer to manage than radioactive materials because X-rays are generated electrically. The radiation stops immediately when the power is turned off. Unlike radioactive isotopes, X-rays do not produce residual radioactivity in the inspected objects, making them safe for industrial use.
These properties of X-rays are widely used not only in the medical field but also in the industrial field, and non-destructive inspection using X-rays allows us to observe the inside of parts and products without breaking or disassembling them. Matsusada Precision manufactures and sells the X-ray non-destructive inspection systems introduced in the following pages to the world.
List of Matsusada Precision's X-ray non-destructive inspection systems
X-ray non-destructive inspectionRelated Technical Articles
Recommended products
Matsusada Precision's high-performance products
Reference (Japanese site)
- Japanese source page 「放射能と放射線、放射性物質はどう違う?」
(https://www.matsusada.co.jp/column/radioactivity_radiation.html) - 環境省ホームページ: 「放射線はどこで生まれる?」
(https://www.env.go.jp/chemi/rhm/h30kisoshiryo/h30kiso-01-03-01.html) - 環境省ホームページ: 「放射線・放射能・放射性物質とは」
(https://www.env.go.jp/chemi/rhm/h30kisoshiryo/h30kiso-01-01-01.html)






