Access to sanitary water remains a global challenge, making clean water technologies increasingly vital. Furthermore, high-purity water is essential for industries such as semiconductor manufacturing, precision equipment cleaning, and chemical production. This article provides an overview of water treatment methods and recent technological advancements.

What is Water Treatment?
Water treatment is the process of removing impurities from water. Even water that appears clean can contain a variety of impurities. Impurities can be broadly divided into the following five categories.
Particulates
Suspended solid particles such as dust, clay, and iron sand.
Microorganisms
Biological contaminants including bacteria, viruses, and other microbial life.
Organic Substances
Dissolved organic matter derived from decomposing plant material (such as algae), organic detergents, or fertilizers.
Inorganic Salts
Dissolved inorganic minerals, including calcium (hardness components), sodium, and iron.
Gases
Atmospheric gases dissolved in water, such as carbon dioxide, oxygen, and hydrogen sulfide.
Water treatment is the process of removing the above impurities to obtain water for a specific purpose.
Purpose of Water Treatment
There are three main types of water treatment objectives.
Treating wastewater before returning it to the environment (Sewage Treatment)
This is the process of purifying wastewater. It removes substances and foreign substances that are harmful to nature from factory effluent and domestic wastewater.
Making water for domestic use from river water and groundwater (water purification treatment)
River water and groundwater also contain various impurities. It removes bacteria and harmful substances from water to produce drinking and safe water for daily use.
Industrial
In some cases, industrial applications require water with even fewer impurities than domestic water, known as pure water. One of the industrial water treatment processes is carried out to obtain this pure water.
Ultrapure water with particularly few impurities is produced for industrial use, which is used for manufacturing semiconductors and precision machinery.
Wastewater treatment represents the largest and most common application of these technologies. To create domestic water, river water is treated at water purification plants, and seawater is desalinated.
Water treatment methods
Water treatment methods can be divided into two main categories.
Physicochemical treatment
Physicochemical treatments can be divided into two categories: physical and chemical. Physical treatment is a method of separating impurities by physical characteristics such as the size and specific gravity of the pollutant. Chemical treatment is a treatment method in which impurities are separated by chemical properties. Each process involves the following techniques
Physical treatment: sedimentation and flotation separation, filtration
Chemical treatments: ion exchange, oxidation (ozone), electrodialysis, electrolysis
Biological Treatment
Biological treatment is a method of using microorganisms such as bacteria to utilize the action of decomposition or conversion by microorganisms, or to destroy pathogenic organisms. Aeration tanks used to treat sewage are another typical example of biological treatment. Biological treatment also includes the treatment of organisms in water, such as the sterilization of pathogens.
Evolving Water Treatment
The development of the semiconductor and precision machinery industries has led to growth in demand for ultrapure water used in these industries. With it, water treatment technology has also evolved. Here are three relatively new water treatments.
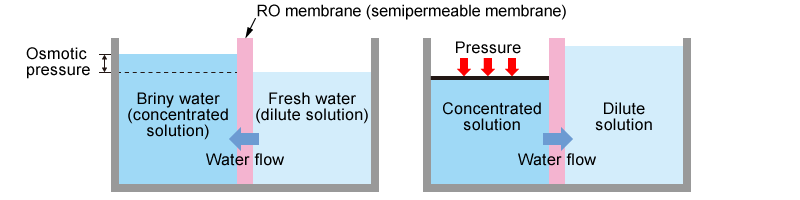
RO Membrane
Reverse Osmosis (RO) is a water treatment method that uses a semipermeable membrane, also called reverse osmosis membranes. When saltwater and freshwater are separated by a semipermeable membrane, the freshwater naturally permeates through the membrane to the saltwater side. Osmotic pressure then occurs on both sides of the membrane.
However, if pressure greater than the osmotic pressure is applied to the saltwater side, reverse osmosis occurs, and water moves from the saltwater side to the freshwater side. This method was originally used to desalinate seawater to make freshwater, but has recently been used in the ultrapure water production system and water recovery fields.
Ion exchange
The ion exchange method in water treatment is a process for obtaining pure water by having impurity ions adsorbed onto ion exchange resins.
There are two types of ion exchange resins: cation exchange resins and anion exchange resins. Cation exchange resins adsorb cations (e.g., Ca2+, Na+) from the water, while anion exchange resins adsorb anions (e.g., Cl-, SO42-). In exchange for adsorbing these impurity ions, the resins release hydrogen ions (H+) and hydroxide ions (OH-) that were bonded to them, resulting in pure water that is almost free of ions.
This method ceases to function when the ion exchange resins become saturated with impurity ions. Therefore, it is necessary to periodically regenerate the resins with chemicals such as acids and alkalis to restore their ion exchange capacity.
EDI (Electro-deionization)
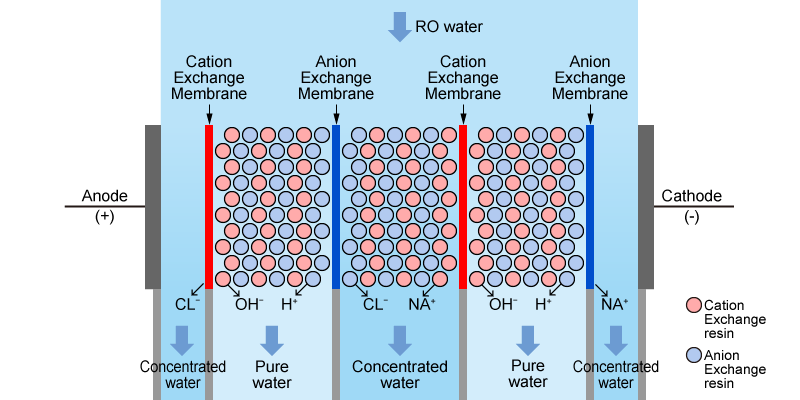
EDI (Electro-deionization), operates as a module composed of multiple membranes.
An EDI module has a structure where cation exchange membranes and anion exchange membranes are arranged alternately between an anode (positive electrode) and a cathode (negative electrode), with the spaces between them filled with ion exchange resins.
When a direct current (DC) is passed between the anode and cathode, anions, such as chloride ions, move toward the anode, and cations, such as sodium ions, move toward the cathode. The cation and anion exchange membranes are structured to selectively allow only their respective ions (cations and anions) to pass through.
This process creates alternating compartments of concentrated ions and diluted ions between the membranes. Pure water is obtained by collecting the water that has passed through these dilution compartments. Since the resins are regenerated electrically and automatically without the use of chemicals, continuous operation is possible.
CEDI (Continuous Electro-deionization)
CEDI (Continuous Electro-deionization) is an advanced, chemical-free water purification technology. Unlike traditional ion exchange, it eliminates the need for periodic resin regeneration using chemicals. CEDI is primarily used in the pharmaceutical and semiconductor industries where continuous production of high-purity water is required.
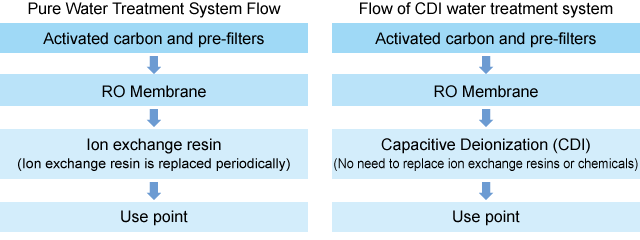
EDI and CEDI are electro-deionization technologies that use a direct current power source to produce pure water continuously. The advantage is that no chemicals are used, so there is no need for wastewater treatment. Ion exchange membranes used in EDI and CEDI are also used in metal recovery applications as well as in the production of pure water.
The voltage and current requirements for the DC power supply in EDI and CEDI systems depend on the size of the ion exchange membrane, typically ranging from 300V to 500V and from several amperes to 10A. The DC power supplies for these systems typically operate in Constant Current (CC) mode to maintain stable ion transfer.
Matsusada Precision offers a wide range of high-performance DC power supplies optimized for EDI and CEDI applications, ensuring the precise control and reliability required for advanced water treatment.
Reference (Japanese site)
- 栗田工業株式会社 - 水処理とはなにか
(https://kcr.kurita.co.jp/wtschool/002.html) - 栗田工業株式会社 - 逆浸透現象を利用したRO膜
(https://kcr.kurita.co.jp/wtschool/008.html) - セイスイ工業株式会社 水処理とは?種類や仕組みの基礎知識を解説
(https://seisui-kk.com/column/ew4k9j5kz) - 室町ケミカル EDIの基礎知識
(https://www.muro-chem.co.jp/media/knowledge/edi)
Recommended products
Matsusada Precision provides solutions for DC power supplies for EDI and CEDI processes in water treatment.






